Detection is based on simple thresholding of acoustic response, making detection real-time. With this real-time detection, tumor cells can be immediately directed into individual vials for later post processing on an individual cell basis. The instrument fits on a benchtop, and the laser path is completely contained while leaving the sample insertion/collection areas exposed for fast and easy testing.
Features
Operation of the instrument is simple, only requiring samples to be loaded by an operator and the software does the rest. Features of the CTCS include:
- Laptop operation with simple and intuitive software
- Real-time detection, sorting, and collection of CTCs (~16 minutes for a 10 ml blood sample)
- Safe, contained laser bench area that is small enough to fit under a hood
- Built-in instrument health monitoring for quality control
- Reliable, air-cooled, diode-pumped laser (no water cooling or flashlamps to change)
- Minimal operator training necessary
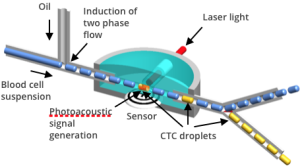
Photoacoustic Detection and Capture of CTCs
The CTCS is not approved by the U.S. FDA and international regulatory agencies for use as a Diagnostic Medical Device.
